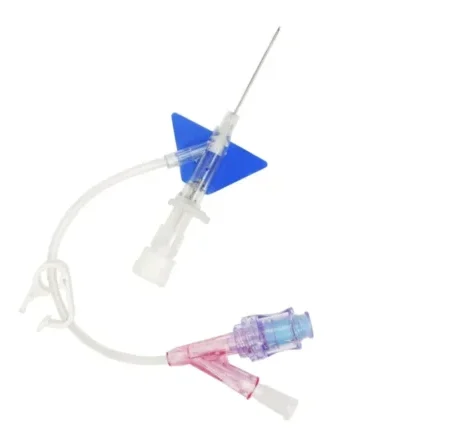
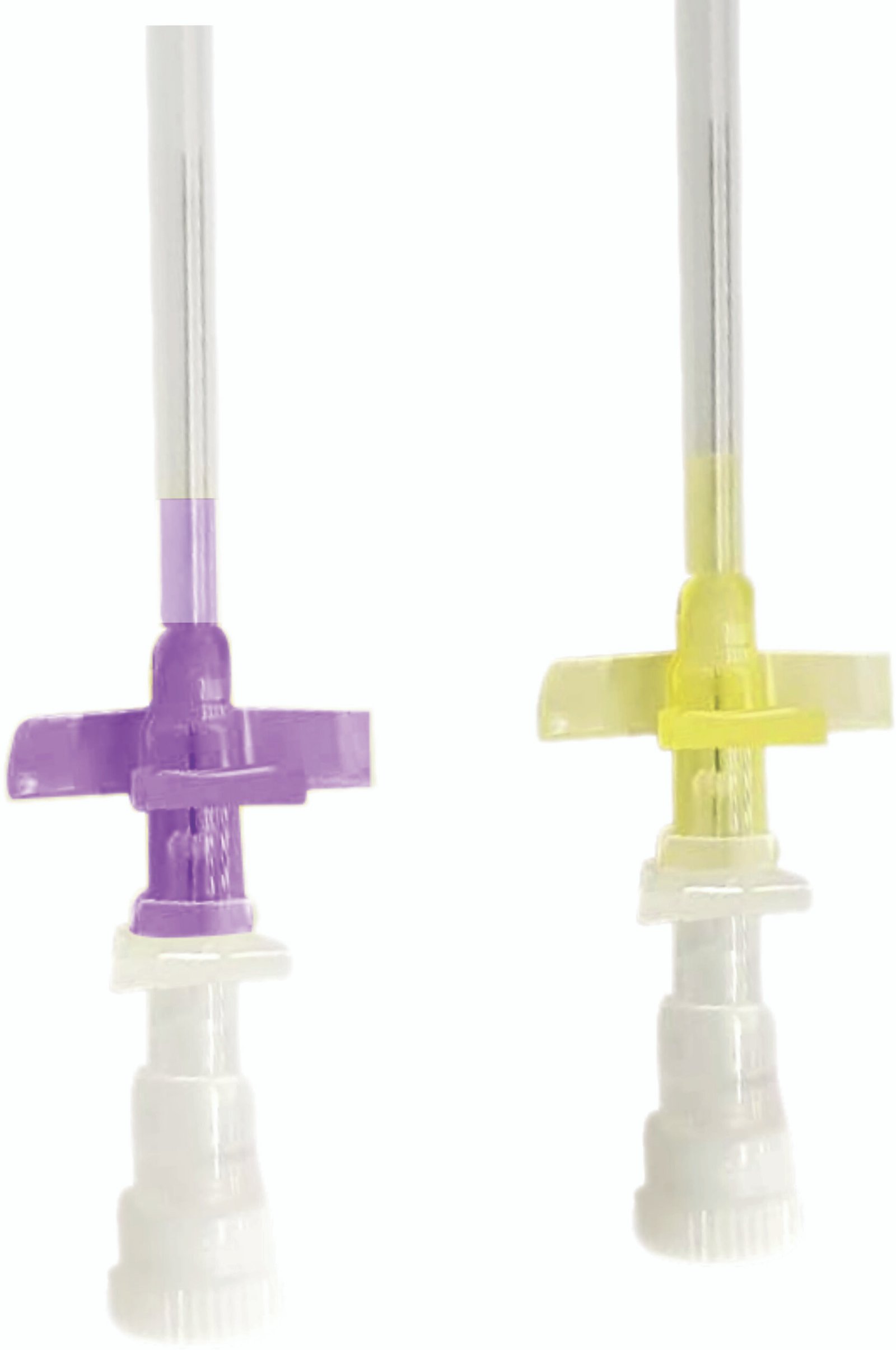
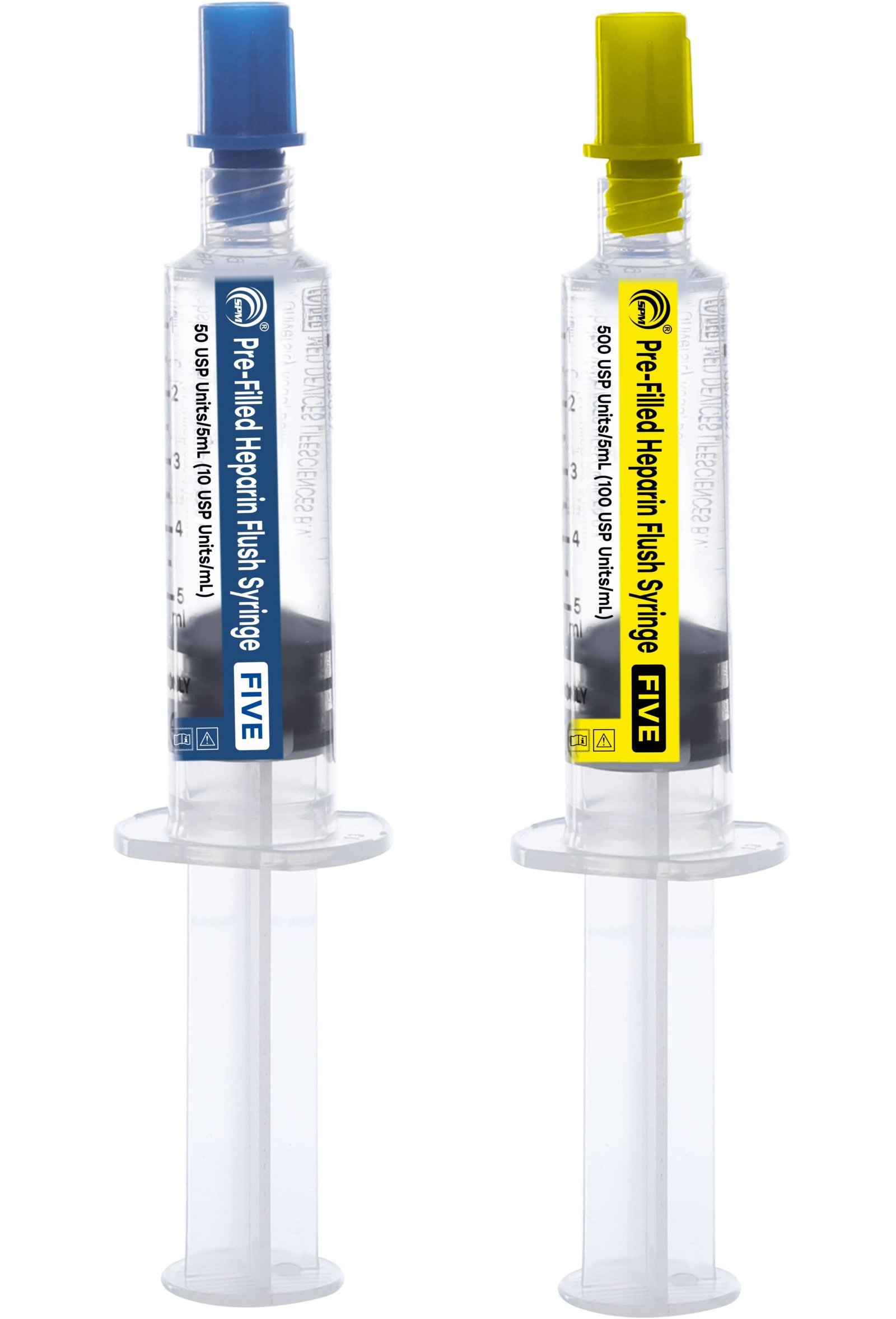
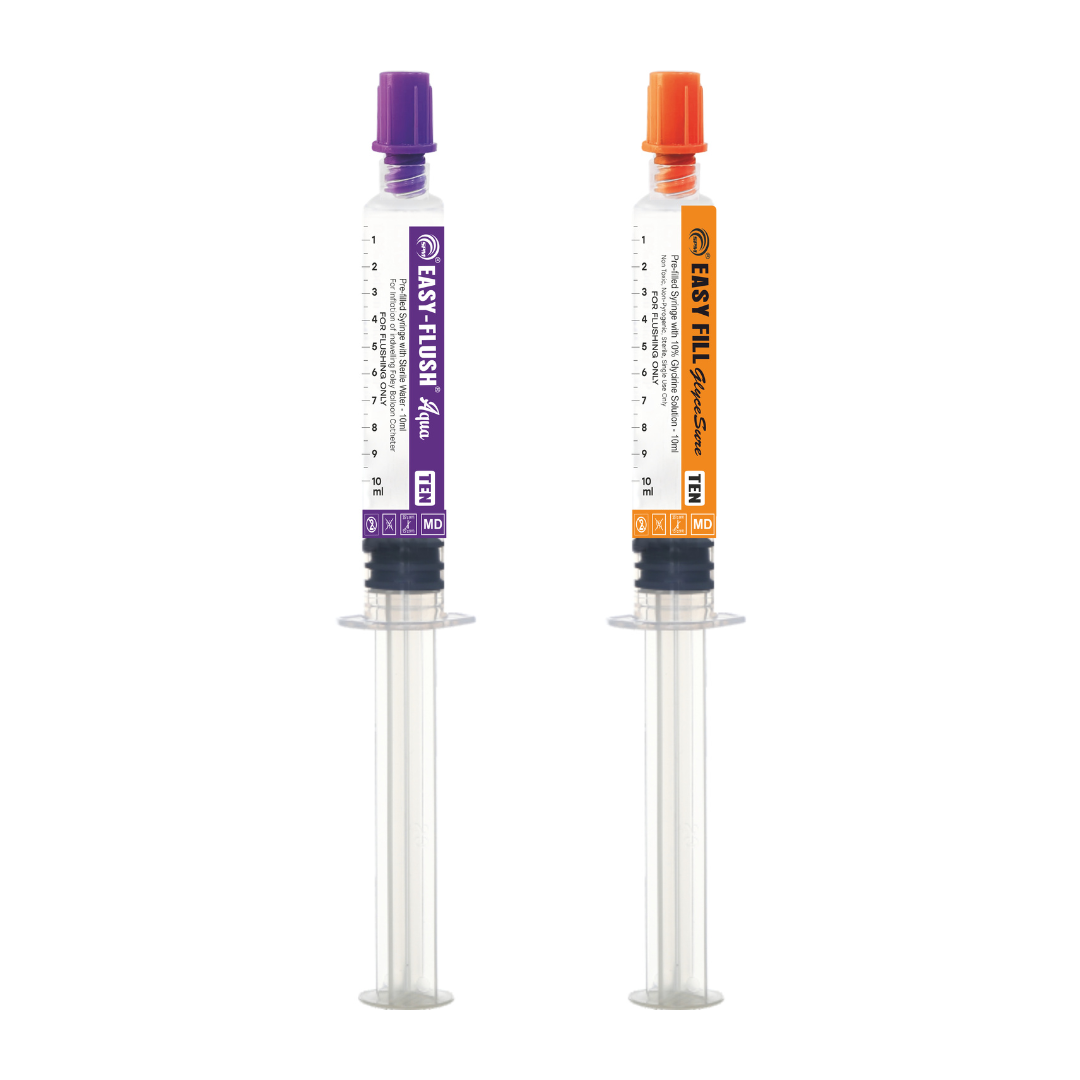
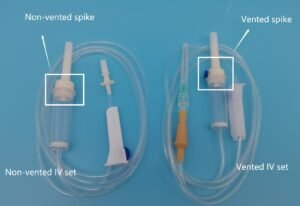
Line Access
Line Conditioning
Line Protection
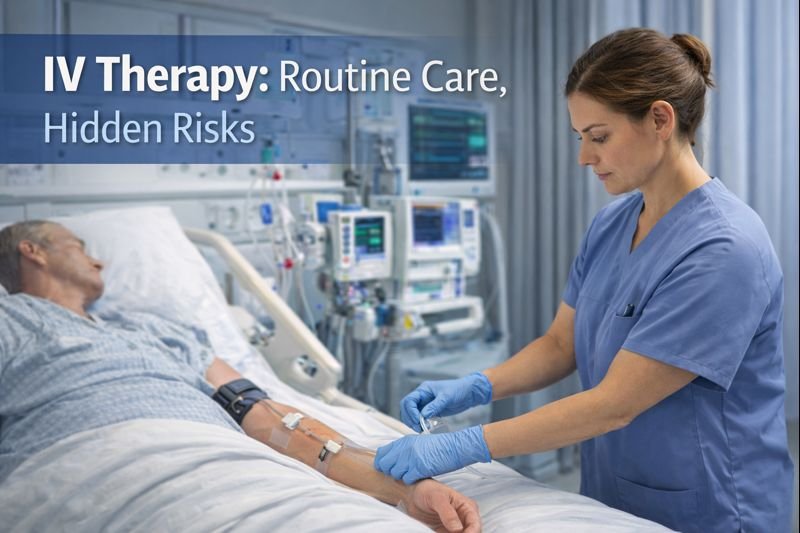
Infusion Therapy
Syringe
About Us

About SPM Medicare
SPM MEDICARE PVT. LTD. has set-up a state-of-the-art manufacturing facility in the National Capital Region (NCR) of India for manufacturing Disposable Medical Devices and Surgical Products. Since the Company’s inception in 2015, SPM Medicare has emerged as one of the fastest growing companies in India in the medical sector, and the Company has won laurels for its commitment to Quality and Service in manufacturing and exporting finest products at competitive prices. This company is also SEDEX approved ensuring social compliance & responsibilities.
The Company has installed top-of-the-line automatic machines in our facility with a quest to achieve optimized manufacturing processes and to achieve highly safe and quality products. The Company manufactures all products in side Clean Room Areas, and we have in-house ETO Sterilizer and dedicated Quality Lab equipped to meet national and international regulatory requirements. In addition to this, ease in the availability of raw material, experienced and dedicated team, and backing from leading financial institutions helps us to operate smoothly and reliably. The Company also values the hygiene and safety of our workers and ensures that they wear recommended safety gear during production.
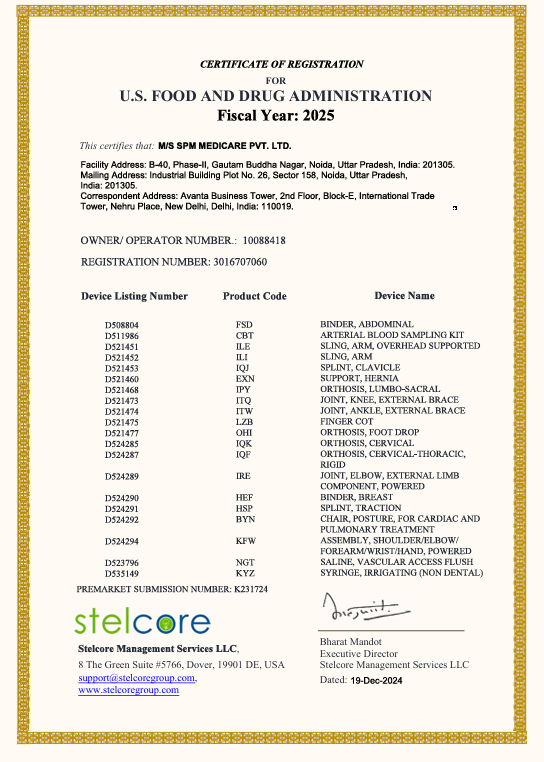
The Company maintains and follows the Quality Management System as per ISO 13485 certified by ITC (Czech Republic). Further our products have also received CE certification from TUV (Germany) and USFDA approved for various products. With above Certifications in place from the most stringent quality authorities, the Company has been able to Market and place our products PAN-India as well as in more than 30 countries around the world.
About SPM Eurocare
The European medical device market demands local presence, regulatory alignment, and responsive partnerships. SPM EuroCare was established to bridge the gap between world-class manufacturing and European healthcare needs.
Latest News
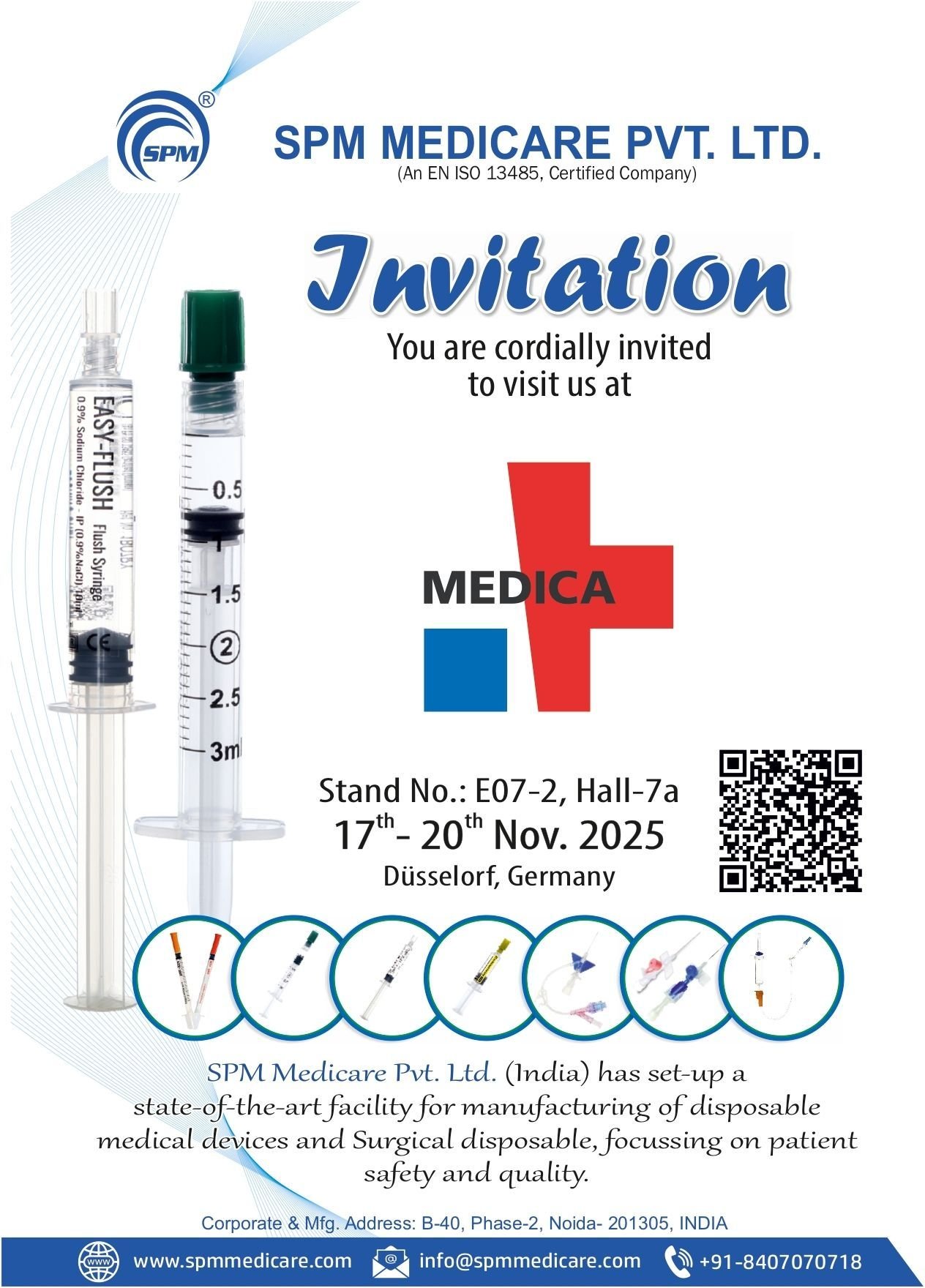
Upcoming Exhibition 2026

Upcoming Exhibition 2026

Last Exhibition 2025

Last Exhibition 2025
Feedback
Vision and Mission
Vision
- To become the most respected organization in the healthcare sector, touching billion lives across the globe by providing safe and quality products and services.
Mission
- To understand the requirements of our customers in the medical and healthcare sectors.
- To design and manufacture high quality products and services as per customer’s requirements.
- To establish an environment wherein we are committed to provide world class care.

Plant & Machineries
A state-of-the-art medical disposable manufacturer with facility that has set up in the heart of the industrial belt of Noida (NCR), designed to incorporate various concepts of ‘Green Building’. The manufacturing facility has a clean & controlled environment for handling raw material and finished goods. Further, hygienic ‘ clean room’ environment has been designed for working areas such as gowning-rooms: Molding sections: assembly section: and packing section, meeting international CE and ISO 14644 standards.

Molding Area

Fully Automatic Assembly Lines
Fully-automatic Insulin Syringe Assembly machine has been specially designed in South Korea to achieve accuracy, quality, and speed in assembling.

IV cannula
IV Sets are manufactured and assembled in Clean Room of class 10,000 by highly skilled and trained manpower to ensure quality and accuracy in assembly process.

ETO Sterilizer Machine
ETO Sterilizer Machine: Efficiently sterilizes medical equipment with ethylene oxide gas, ensuring safety and quality in healthcare facilities.
Article
Education Poster
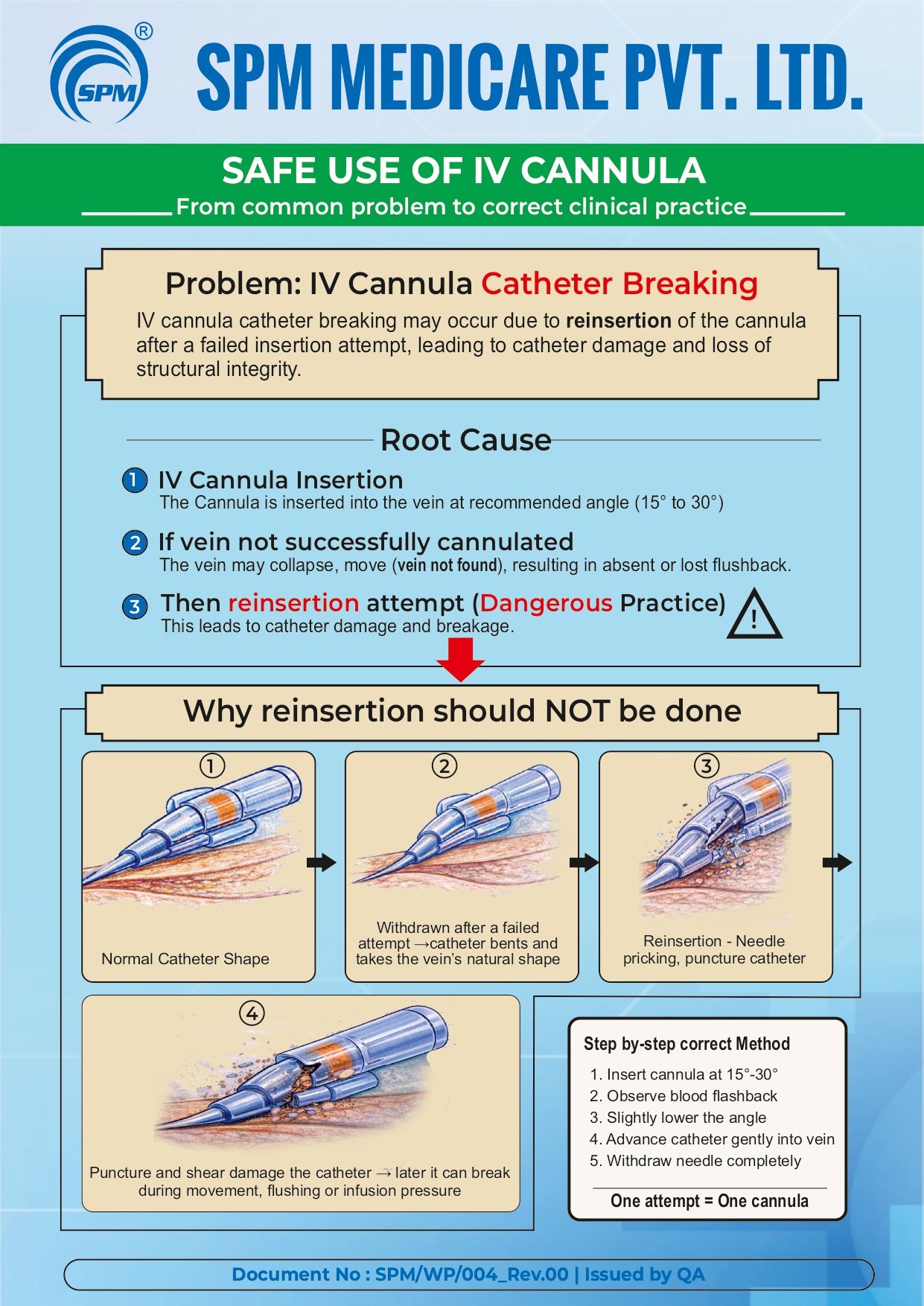
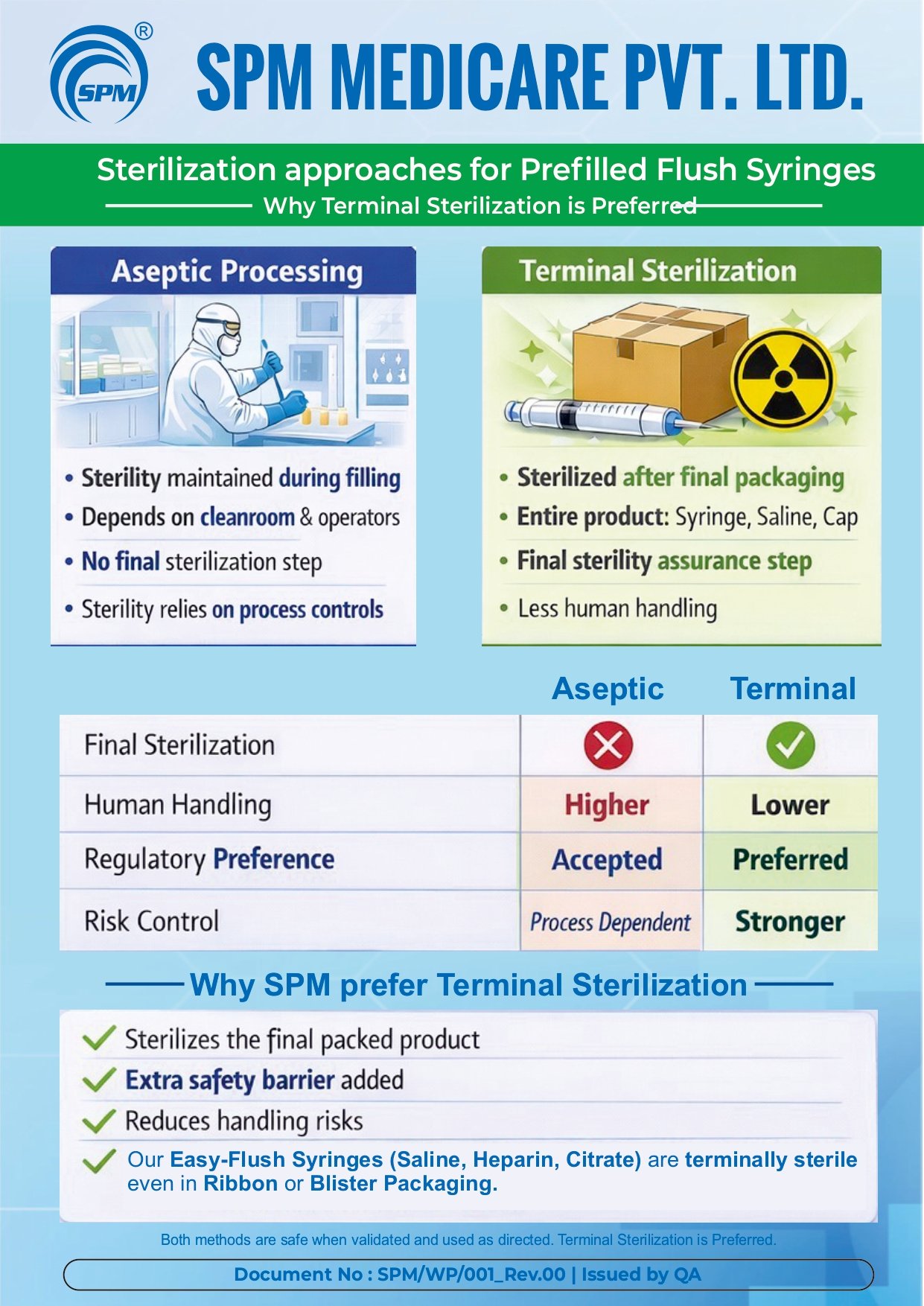
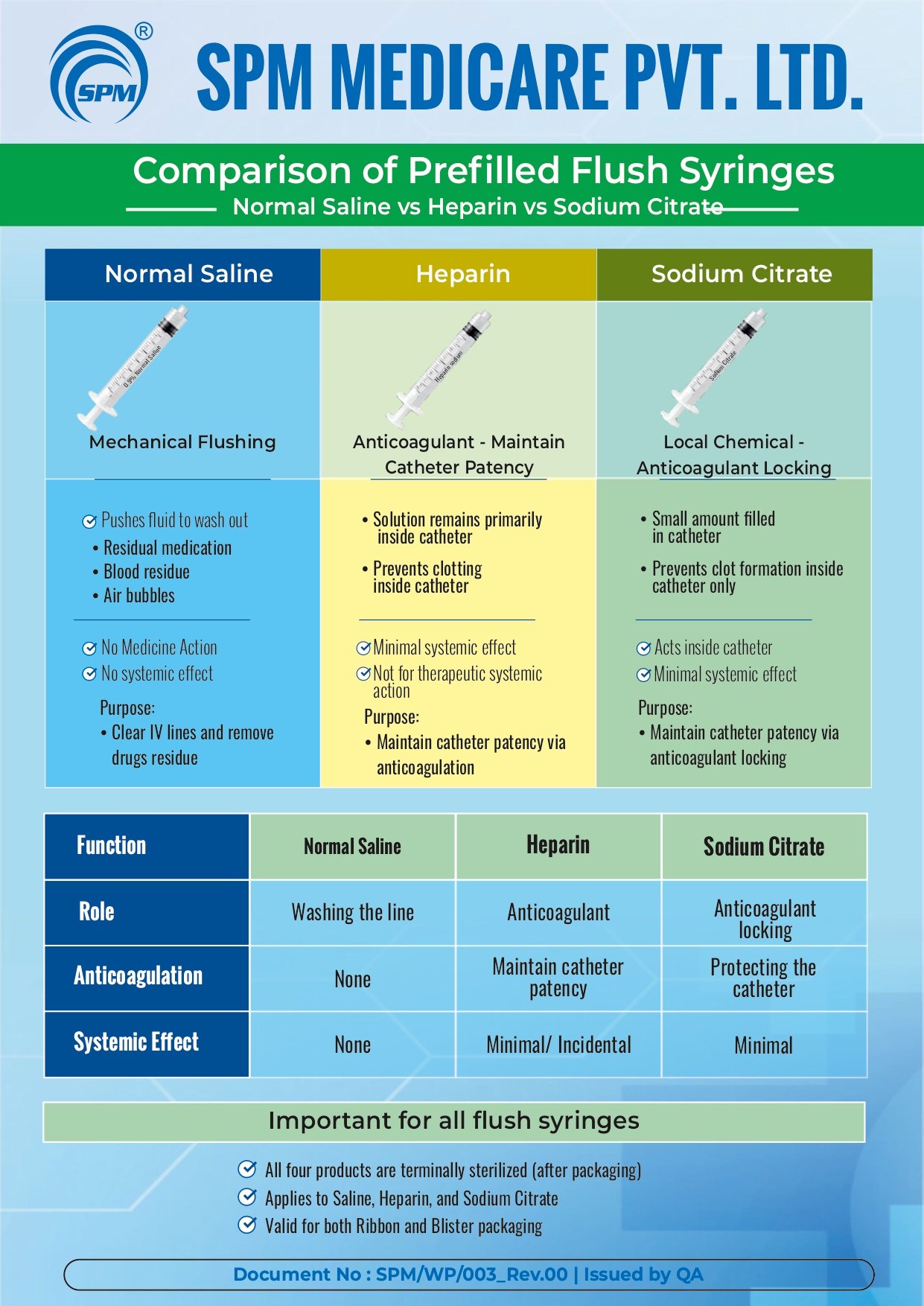
Education Video
Management
A team of hard-working enthusiastic people who helped this product come to life.

Contact Us
Get In Touch
We constantly keep challenging ourselves to deliver the most sincere service to our global clients. Connect with us today to learn more about our products
Let’s Connect With US
Our Gallery